Wetting and Spreading
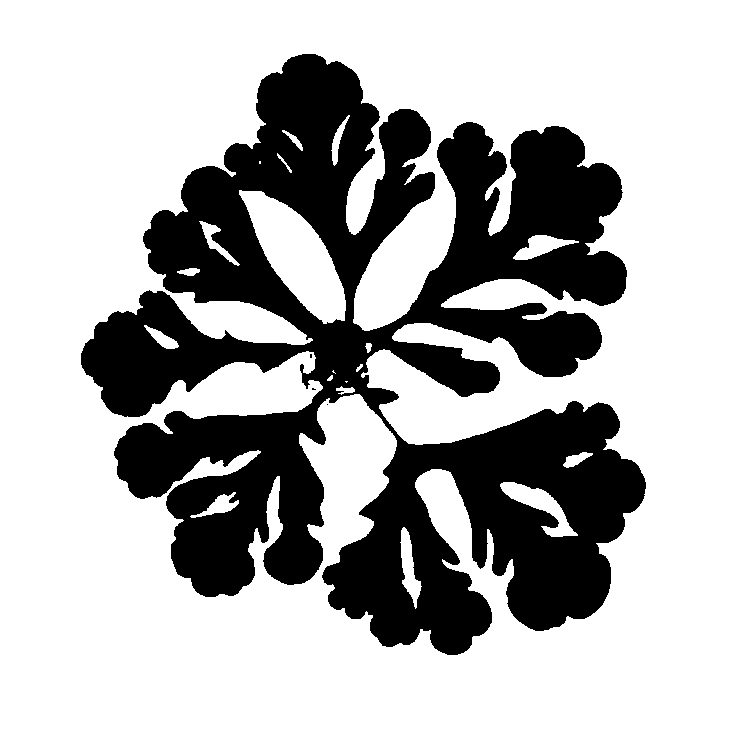
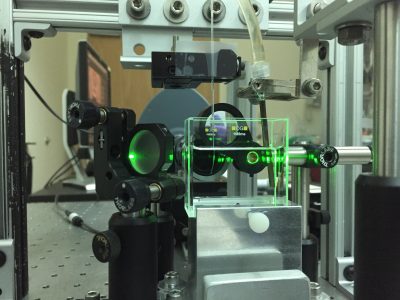
Liquid metals are known for their high surface tension. Nonetheless, by applying a small voltage to Gallium-Indium droplets in solution, the metal’s own oxide can act as a surfactant and allow the droplet to spread. In collaboration with Michael Dickey’s lab (NCSU Chemical Engineering), we have been characterizing a new class of fingering instabilities that appear as a result of these oxides. Intriguingly, while the production of a small amount of oxide can lead to fractal patterns (shown at left), thicker layers of oxide impede spreading by creating a crust that resists spreading. Controlling the competition between surface tension and oxidative (compressive) stresses is important for the development of reconfigurable electronic, electromagnetic, and optical devices that take advantage of the metallic properties of liquid metals.
- Collin B. Eaker, David C. Hight, John D. O’Regan, Michael D. Dickey, Karen E. Daniels. “Oxidation-Mediated Fingering in Liquid Metals.” [arXiv] (to appear in Physical Review Letters)
Biological and polymeric materials with low Young’s modulus are soft enough that capillary forces play a significant role in defining their shape and dynamics. We aim to quantify the nature of the contact line forces between fluids and soft (gel) substrates by directly measuring internal strains in response to external forces and fluids. This work is done in close collaboration with Aaron Bardall and Michael Shearer (NCSU Math) Current researchers: Shih-Yuan Chen